In 1997, SLA surface technology was pioneered by the Swiss medical device manufacturing company Straumann.
Renowned for its proven safety in extensive clinical cases, SLA surface technology stands as the most extensively utilized surface technology worldwide, however, due to surface treatment methods using acids, the possibility of residue and toxicity on the implant surface cannot be completely ruled out. To address this, DENTIS has developed the innovative surface technology, C-SLA™!
Furthermore, contamination that can occur during the production and packaging processes cannot ensure the stability of implants! If contaminated implants of poor quality are implanted in patients, it can lead to fatal consequences. Therefore, the implant production environment must also be meticulously taken care of!

Recognizing these risks, DENTIS innovatively applied Clean-Tech™ to the globally recognized SLA surface, resulting in the development of CLEAN SLA, abbreviated as C-SLA™ surface technology. Through DENTIS’ unique Clean-Tech™, concerns regarding ‘residue’ and ‘toxicity,’ typically associated with the traditional SLA surface treatment processes involving Sandblasting, Large Grit, and Acid Etching, have been eliminated. This advancement has significantly enhanced the clinical safety standards. As a result of these endeavors, ‘C-SLA™’ has achieved an unprecedented outcome with no residue and no lot defects.
The advanced surface technology C-SLA™ is developed with innovations such as ultra-pure, ultra-precision cleaning and sterilization equipment including Vacuum Ultrasonic Wave System, Spherical Wave System, PID System, and more, which have the implants go through 30 steps of cleaning alone.
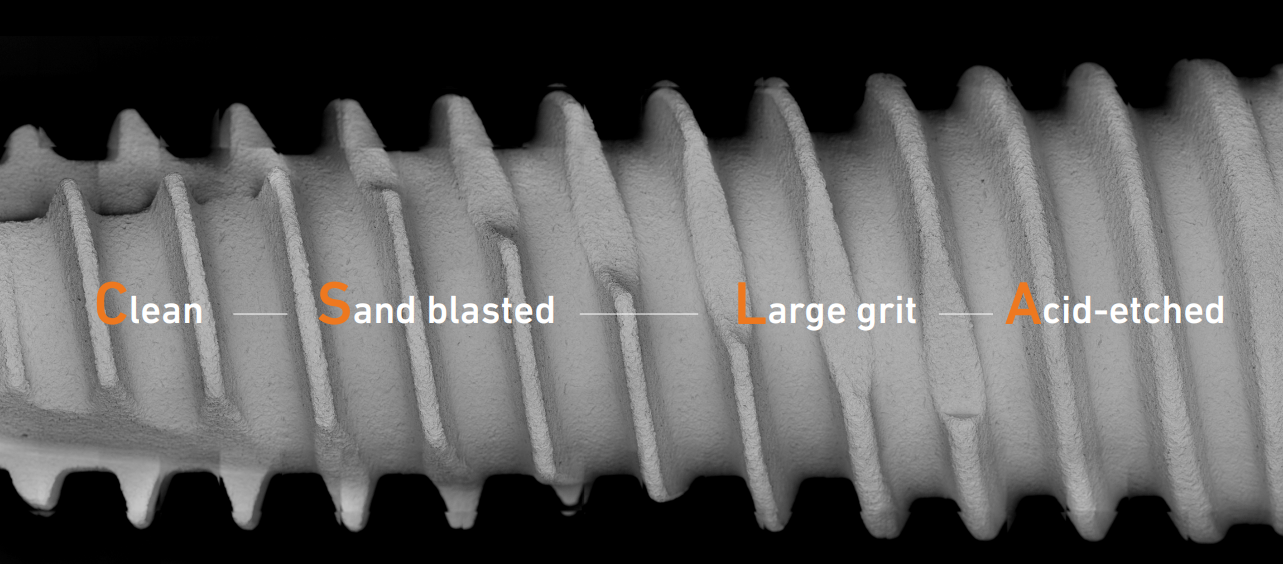
DENTIS implants utilize the C-SLA™ surface technology to boast exceptional safety and quality assurance and has achieve an unprecedented outcome with no reside, no toxicity, and no lot defects.
As a result of our endeavors, the DENTIS SQ Implants, was awarded with the, “Trusted Quality,” certificate from Germany’s Clean Implant Foundation in December 2023, solidifying our position as trustworthy implants.

The Clean Implant Foundation (CIF) is a nonprofit implant quality evaluation organization based in Germany, established in 2016! They rigorously evaluate implant quality according to strict verification procedures and grant certification to only a few implants among hundreds of implant manufacturing companies worldwide. Therefore, the certification of the DENTIS SQ implant by CIF is especially meaningful and significant!
The C-SLA™ surface technology is the culmination of DENTIS’s commitment to clean principles since 2006, offering unparalleled technical prowess and brand value!
DENTIS’s C-SLA™ technology has received high praise in the dental field for completely eliminating residue and toxicity issues, enhancing safety, and delivering high-quality clean implants!
Building on these achievements, DENTIS continues to innovate and aims to grow as a leading company in the global dental market.